Describe the Relationship Between Hydrogen Ions and Ph
On the relationship between potassium and acid-base balance. We know that solutions can be acidic neutral or basic alkaline.
Relationship Between Hydrogen Ions And Ph Definition Properties Ph Scale And Values
Concentration of hydrogen ions increases pH value decreases In an acidic solution the concentration of hydrogen ions depends on the concentration or molarity of the acidic.

. Therefore the more hydrogen ions present the lower the pH. 2 However maintenance of normal extracellular K 35 to 5 mEqL is under two potential threats. It has an even number of hydrogen ions H.
PH is the measure of hydrogen ions in a solution. What characteristic of carbon atoms allows the formation of a large number of compounds. The Relationship Between pH and Hydrogen Ion.
PH -log 10 H aq Therefore there is a direct relationship between pH and the concentration of. The overall concentration of hydrogen ions is inversely related to its pH and can be measured on the pH scale Figure 1. 1 Maintenance of extracellular K concentration within a narrow range is vital for numerous cell functions particularly electrical excitability of heart and muscle.
Summarized from Lee Hamm l Hering-Smith K Nakhoul N. The lower the pH the more concentrated the hydrogen ions because pH measures the negative log of hydrogen ion concentration meaning 01 ion concentration is one on the pH scale and 0001 is three. PH -log10H where H is the concentration of hydrogen ions.
The pH level or possible level of hydrogen in your body is determined by the food and type of drink you consume. A base is a solution with a larger hydroxide ion concentration than the hydrogen ion concentration. A solution with a concentration of hydrogen ions higher than 10-7molL is acidic and a solution with a lower concentration is alkaline another way to say basic.
Hydrogen ion concentration is inversely related to pHthe higher the hydrogen ion concentration the lower the pH and the more acidic the solution. This concentration can range over a tremendous range from 10-1 to 10-14. The solution is acidic when the hydrogen ions outnumber the hydroxide ions.
The notion that acid-base and potassium homeostasis are linked is well known. Figure shows the pH values of acidic. Acid-base and potassium homeostasis.
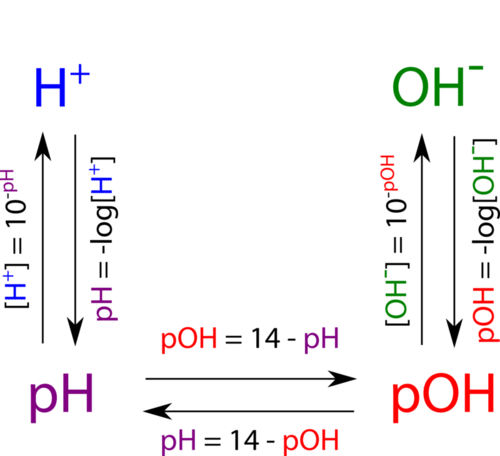
Log H pH The molar concentration of dissolved hydrogen ions in solution is a measure of acidity. So a convenient way to scale down this range is the pH scale which means power of hydrogen. Acids and Bases Numerically.
The pH is the concentration of the hydrogen ions. Conversely the fewer hydrogen ions the higher the pH. Using the formula pH-logH a pH of 7 is neutral a pH less than 7 is acidic and a pH greater than 7 is.
An ion of one hydrogen and one oxygen OH created by the breaking up of a water moleculeloss of a hydrogen during the mixing of a solution. The pH is the potential of hydrogen H ions in a solution while the pOH is the potential of hydroxide OH ions in a solution. The pH measures the acidity of a solution while the pOH measures the alkalinity of a solution.
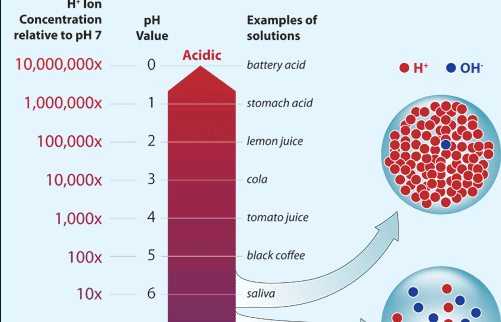
A solution with the pH 3 is 1000 times less acid than a solution with the pH 1. The greater the concentration the greater the acidity. The pH scale is logarithmic and shows the solutions concentration of hydrogen ions inversely.
The effects of acid-base balance on serum potassium are well known. A solution that has a pH of around 7. PH -log 10 a H Where a is the activity.
Differences in pH are in orders of ten in relation to the hydrogen ion concentration. PH is the solutions acidity. Here is an example.
Describe the relationship between the Hydrogen Ions H and pH. Students of laboratory medicine will learn that in general acidemia. Describe the relationship between hydrogen ions H and pH.
How is pH related to a solutions acidity. They are like opposites of each other. The relationship between pH values and concentration of hydrogen ions is given below.
Not all solutions are neutral when this happens the hydrogen ion concentration is greater than the hydroxide ion concentration and is known as an acidic solutionWhich means that H is greater than 10 x 10-7 M. AS SOON AS POSSIBLE. Describe the relationship between hydrogen ions and pH.
H OH-Kw10-14 fixed at 25 o C. 4 rows Hydrogen ion concentration. From this equation we can see that an increase of hydrogen ions will lower the pH and a decrease of hydrogen ions will raise the.
Contains more hydrogen ions H than hydroxide ions OH. This calculation is based on a 0 to 14 scale. Seminars in Nephrology 2013.
One connection between pH and the concentration of hydrogen ions is that together they allow the use of a logarithmic scale. Which means that H is. Acids have a pH lower that 7.
Then the pH is the logarithm of the inverse of the hydrogen ion concentration. The pH -log of hydrogen ion concentration The more acid a solution is the higher is its concentration of H ions and the lower is its pH. A solution that is between 0-6 on the pH Scale.
How is pH related to a solutions acidity. Since the concentration of the hydrogen ions is often very low ion activity is considered as equal to the concentration of hydrogen ions. If the situation is reversed the solution is alkaline.
For any solution the following relationship between the densities of hydrogen ions H and hydroxide ions OH- is observed if the temperature does not change.

Lesson Explainer The Ph Scale Nagwa
What Is Ph College Of Agriculture Forestry And Life Sciences Clemson University South Carolina
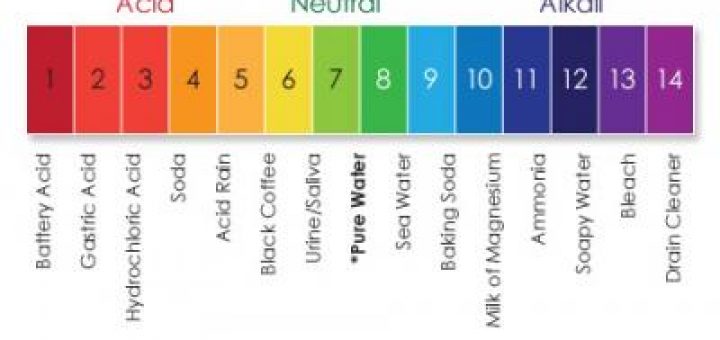
Power Potential Of Hydrogen Hydrogen Ion Concentration Ph Relation Between Ph Enzyme Activity Science Online

Ph Value And Corresponding H Ion Concentration Download Table
8 6 The Ph Concept Chemistry Libretexts

How To Calculate Ph From The Hydrogen Ion Concentration Chemistry Study Com

Relationship Between Ph Values And Molarity Of Acids And Alkalis A Plus Topper

Lesson Explainer The Ph Scale Nagwa

How To Calculate Hydrogen Ion Concentration From Ph Youtube
Concentration Of Hydrogen Ions Horiba
Small Drop In Ph Means Big Change In Acidity Woods Hole Oceanographic Institution

Hydrogen Ion Concentration An Overview Sciencedirect Topics
Relationship Between Hydrogen Ions And Ph Definition Properties Ph Scale And Values

The Ph Scale Chemistry For Non Majors
Is Ph The Measurement Of Hydrogen Ion Concentration Or Ion Activity

What Is Ph Definition Overview Expii
Relationship Between Hydrogen Ions And Ph Definition Properties Ph Scale And Values


Comments
Post a Comment